Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
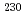 Th-
Th- U age dating of bulk uranium for nuclear forensics
U age dating of bulk uranium for nuclear forensicsGaffney, A.*; Hubert, A.*; Kinman, W. S.*; Magara, Masaaki; Okubo, Ayako; Pointurier, F.*; Schorzman, K. C.*; Steiner, R. E.*; Williams, R. W.*
Journal of Radioanalytical and Nuclear Chemistry, 307(3), p.2055 - 2060, 2016/03
Times Cited Count:22 Percentile:89.61(Chemistry, Analytical)In and inter-laboratory measurement comparison study, four laboratories (LLNL, LANL, CEA, JAEA) determined 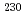 Th-
Th- U model ages of uranium certified reference material NBL U050 using isotope dilution mass spectrometry. The model dates determined by the participating laboratories range from 9 March 1956 to 19 October 1957, and are indistinguishable given the associated measurement uncertainties. These model ages are concordant with to slightly older than the known production age of NBL U050, indicating unsufficient purification of U050.
U model ages of uranium certified reference material NBL U050 using isotope dilution mass spectrometry. The model dates determined by the participating laboratories range from 9 March 1956 to 19 October 1957, and are indistinguishable given the associated measurement uncertainties. These model ages are concordant with to slightly older than the known production age of NBL U050, indicating unsufficient purification of U050.
 Th/
Th/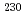 Th ratios
Th ratiosOkubo, Ayako; Shinohara, Nobuo; Magara, Masaaki
no journal, ,
The uranium age-dating is a nuclear forensics analysis techniques, by measuring the 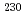 Th /
Th /  U isotope ratio in uranium sample, to estimate the elapsed time from being separated and purified. We have determined
U isotope ratio in uranium sample, to estimate the elapsed time from being separated and purified. We have determined 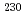 Th and
Th and  U using isotope dilution mass spectrometry method, it has conducted for uranium age-dating. Act to perform the isotope analysis after the addition a known quantity of the isotope (spike) to the sample, after spiking, analytical sample is assumed to be in the state of isotopic equilibrium. However, the samples which have complex speciation of uranium and thorium are analyzed, may not sufficiently reach to the state of isotopic equilibrium has been suggested from the experimental results. In this study, without the addition of a spike, from the measurement results of the uranium isotope ratios and thorium isotope ratio in the sample, it was investigated a method for calculating the
U using isotope dilution mass spectrometry method, it has conducted for uranium age-dating. Act to perform the isotope analysis after the addition a known quantity of the isotope (spike) to the sample, after spiking, analytical sample is assumed to be in the state of isotopic equilibrium. However, the samples which have complex speciation of uranium and thorium are analyzed, may not sufficiently reach to the state of isotopic equilibrium has been suggested from the experimental results. In this study, without the addition of a spike, from the measurement results of the uranium isotope ratios and thorium isotope ratio in the sample, it was investigated a method for calculating the 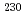 Th /
Th /  U isotope ratio. The state of radioactive equilibrium between
U isotope ratio. The state of radioactive equilibrium between  U and
U and  Th in the sample was utilized in the
Th in the sample was utilized in the  Th /
Th / 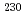 Th method. The
Th method. The 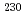 Th /
Th /  U ratio was calculated using measured
U ratio was calculated using measured  Th /
Th / 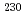 Th isotope ratio,
Th isotope ratio,  U /
U /  U isotope ratio and
U isotope ratio and  Th /
Th /  U ratio in the radioactive equilibrium.
U ratio in the radioactive equilibrium.